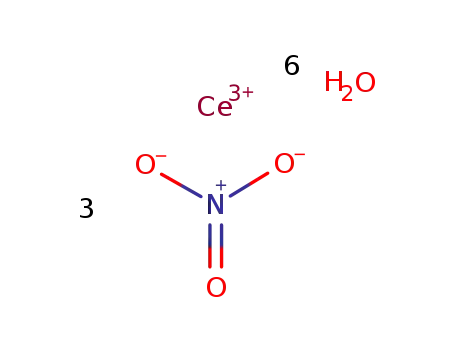
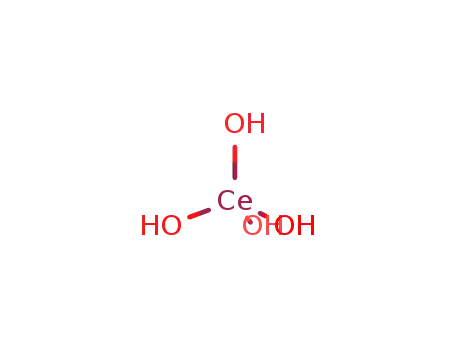
Syntheses and crystal structures of hydrated ternary cerium sulfates: Mixed-valence K5Ce2(SO4)6· H2O and K2Ce(SO4)3·H 2O
Casari, Barbara M.,Langer, Vratislav
, p. 1055 - 1061 (2007)
A new chemical and structural interpreta...
Hydrolysis of lanthanide dicarbides. Rates of reaction with water
McColm, I. J.,Quigley, T. A.,Bourne, D. R.
, p. 191 - 198 (1991)
The kinetics of the reaction between liq...
Influence of the incorporation of CeO2 nanoparticles on the ion exchange behavior of dodecylsulfate doped polypyrrole films: Ac-electrogravimetry investigations
Benmouhoub,Agrisuelas,Benbrahim,Pillier,Gabrielli,Kadri,Pailleret,Perrot,Sel
, p. 270 - 280 (2014)
Cerium oxide (CeO2) nanoparticles (NPs) ...
Synthesis methods for Ce(CrO4)2 · xH 2O and crystal structures of K2CrSO7, (NH 4)2Cr2O7 and Na2Cr 2O7 · 2H2O
Casari, Barbara M.,Eriksson, Annika K.,Langer, Vratislav
, p. 771 - 777 (2007)
New and quick methods to synthesize Ce(C...
Study of the Ru/Ce system in the oxidation of carbon black and volatile organic compounds
Aouad,Saab,Abi-Aad,Aboukais
, p. 835 - 840 (2007)
The relationship between the state of Ru...
Facile synthesis of ceria nanoparticles by precipitation route for UV blockers
Anupriya,Vivek,Subramanian
, p. 406 - 410 (2014)
Homogeneous ceria (CeO2) nano particles ...
Dipotassium tetrachromate(VI), K2Cr4O13
Casari, Barbara M.,Langer, Vratislav
, p. i117-i119 (2005)
The structure of dipotassium tetrachromi...
Cerium doped magnetite nanoparticles for highly sensitive detection of metronidazole via chemiluminescence assay
Haddad Irani-nezhad, Mahsa,Hassandoost, Ramin,Joo, Sang Woo,Khataee, Alireza,Orooji, Yasin,Rahim Pouran, Shima
, (2020)
Cerium doped magnetite nanoparticle (CDM...
New structure type among octahydrated rare-earth sulfates, β-Ce 2(SO4)3·8H2O, and a new Ce 2(SO4)3·4H2O polymorph
Casari, Barbara M.,Langer, Vratislav
, p. 1074 - 1081 (2007)
Syntheses, crystal structures and therma...
Thermochemical study of tetravalent metal sulfate tetrahydrates: A4+(SO4)2(H2O)4 (A4+?=?Zr, Ce, U)
Zhang, Lei,Lobeck, Haylie L.,Dzik, Ewa A.,Sigmon, Ginger E.,Burns, Peter C.
, p. 56 - 60 (2019)
The syntheses, characterization, and cal...
Catalysis on Ruthenium Clusters Supported on CeO2 or Ni-Doped CeO2: Adsorption Behavior of H2 and Ammonia Synthesis
Izumi, Yasuo,Iwata, Yasuhiro,Aika, Ken-ichi
, p. 9421 - 9428 (1996)
Catalysis on Ru clusters supported on Ce...
A pseudo-merohedrally twinned rare-earth sulfate: K6[Ce(HSO 4)2(SO4)4]·-H2O, a novel structure type
Casari, Barbara M.,Langer, Vratislav
, p. i25-i27 (2007)
A novel structure type of an acidic rare...
Oxidation of ketones by ceric perchlorate catalysed by iridium(III)
Tandon, Praveen K.,Sahgal, Sumita,Singh, Alok K.,Gayatri,Purwar, Manisha
, p. 83 - 88 (2005)
In search of economical and effective ca...
Influence of molybdenum on ceria activity and CO2 selectivity in propene total oxidation
Flouty,Abi-Aad,Siffert,Aboukais
, p. 219 - 226 (2004)
A total oxidation of propene into CO2 is...
Hydrolysis of lanthanide dicarbides. Rates of reaction with water vapour
McColm, I. J.,Quigley, T. A.
, p. 347 - 360 (1991)
The kinetics of the reaction between wat...
Size Dependence of Lattice Parameter and Electronic Structure in CeO Nanoparticles
Beck, Aaron,Bonani, Walter,Eloirdi, Rachel,Engelhard, Mark H.,Gouder, Thomas,Guo, Xiaofeng,Kriegsman, Kyle W.,Kvashnina, Kristina,Martin, Philippe,Popa, Karin,Prieur, Damien,Scheinost, Andreas C.,Vitova, Tonya,Walter, Olaf
, (2020)
Intrinsic properties of a compound (e.g....
Pd/REOs catalysts applied to the Suzuki-Miyaura coupling. A comparison of their catalytic performance and reusability
Del Zotto, Alessandro,Colussi, Sara,Trovarelli, Alessandro
, p. 275 - 283 (2017/09/30)
Two new palladium catalysts based on rar...