Product Details
CAS: 206996-60-3
MF: Ce(C2H3O2)3·4H2O
Appearance: white to yellow powder
Packing: 25kg/ bag, 50kg/ bag.
Purity: CeO2/TREO≥99.99%
Chinese Factory Supply High Purity 99% 206996-60-3 In Stock with Competitive Price
- Molecular Formula:Ce(C2H3O2)3·4H2O
- Molecular Weight:317.25
- Appearance/Colour:white to yellow powder
- Melting Point:686 °C(lit.)
- PSA:0.00000
- Density:4.46 g/mL at 25 °C(lit.)
- LogP:2.06850
Cerium Acetate(Cas 206996-60-3) Usage
Samarium chloride anhydrous is used for the preparation of Samarium metal, which has a variety of uses, notably in magnets. Anhydrous SmCl3 is mixed with Sodium Chloride or Calcium Chloride to give a low melting point eutectic mixture. Electrolysis of this molten salt solution gives the free metal.
InChI:InChI=1/ClH.6H2O.Sm/h1H;6*1H2;/q;;;;;;;+3/p-1
206996-60-3 Relevant articles
Effect of reaction temperature on the chlorination of a Sm 2O3-CeO2-C mixture
Esquivel,Bohé,Pasquevich
, p. 47 - 55 (2005)
The chlorination of a Sm2O3-CeO2-C mixtu...
SYNTHESIS AND STRUCTURAL STUDY OF SAMARIUM HEXACYANOFERRATE (III) TETRAHYDRATE, SmFe(CN)6 multiplied by (times) 4H2O.
Mullica,Perkins,Sappenfield,Grossie
, p. 9 - 15 (1988)
Single crystals of SmFe(CN)//6 multiplie...
Synthesis, structure and luminescence properties of samarium (III) and dysprosium (III) complexes with a new tridentate organic ligand
An, Bao-Li,Gong, Meng-Lian,Li, Ming-Xing,Zhang, Ji-Ming
, p. 1 - 6 (2004)
A novel organic ligand, 6-diphenylamine ...
The first dinitrile frameworks of the rare earth elements: ∞3[LnCl3(1,4-Ph(CN)2)] and ∞3[Ln2Cl6(1,4-Ph(CN) 2)], Ln = Sm, Gd, Tb, Y; access to novel metal-organic frameworks by solvent free synthesis in molten 1,4-benzodinitrile
Hoeller, Christoph J.,Mueller-Buschbaum, Klaus
, p. 10141 - 10149 (2008)
The three-dimensional frameworks ∞3[LnCl...
Electronic polarizability of a Sm3+ ion estimated from refractive indexes and molar volumes of molten SmCl3
Iwadate, Yasuhiko,Shirao, Kazuya,Fukushima, Kazuko
, p. 89 - 91 (1999)
The refractive indexes and the molar vol...
Self-assembled light lanthanide oxalate architecture with controlled morphology, characterization, growing mechanism and optical property
He, Hongmei,Zhang, Youjin,Zhu, Wei,Zheng, Ao
, p. 1546 - 1552 (2011/10/01)
Flower-like Sm2(C2O4)3· 10H2O had been s...
Preparation and characterization of rare earth orthoborates, LnBO 3 (Ln = Tb, La, Pr, Nd, Sm, Eu, Gd, Dy, Y) and LaBO3:Gd, Tb, Eu by metathesis reaction: ESR of LaBO3:Gd and luminescence of LaBO3:Tb, Eu
Velchuri, Radha,Kumar, B. Vijaya,Devi, V. Rama,Prasad,Prakash, D. Jaya,Vithal
, p. 1219 - 1226 (2011/07/09)
Lanthanide orthoborates of composition L...
206996-60-3 Upstream products
-
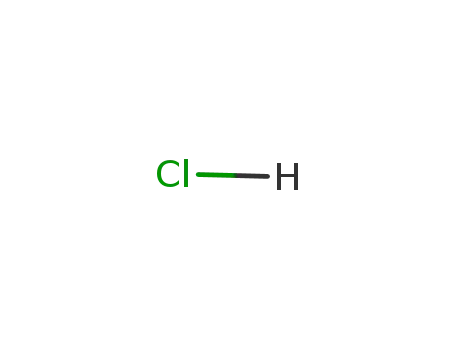
7647-01-0
hydrogenchloride
-
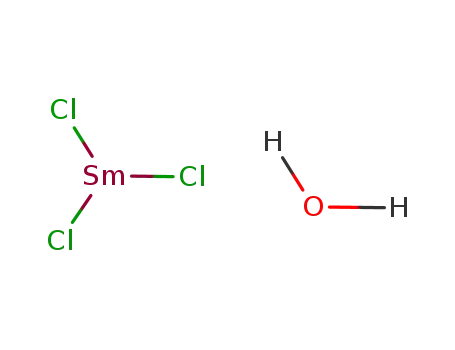
114364-18-0
SmCl3*H2O
-
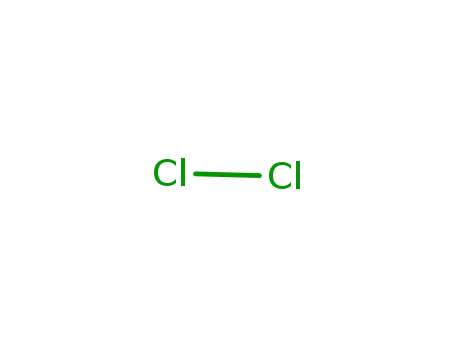
7782-50-5
chlorine
-
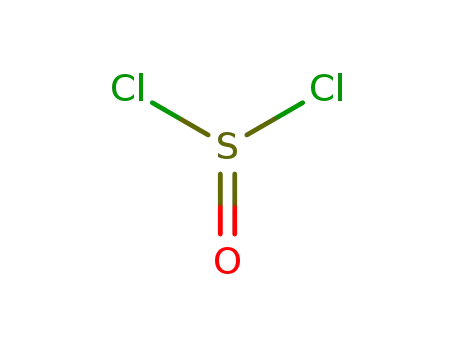
7719-09-7
thionyl chloride
206996-60-3 Downstream products
-
52645-53-1
permethrin
-
7440-19-9
samarium
-
98521-14-3
(SmCl3((C6H11)2PHO)3)