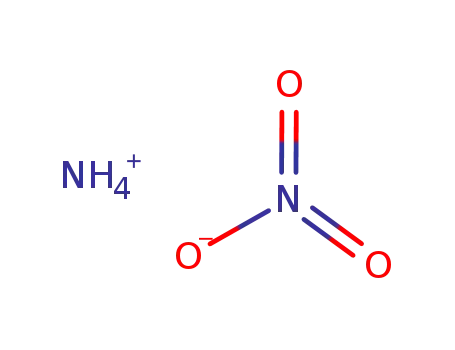
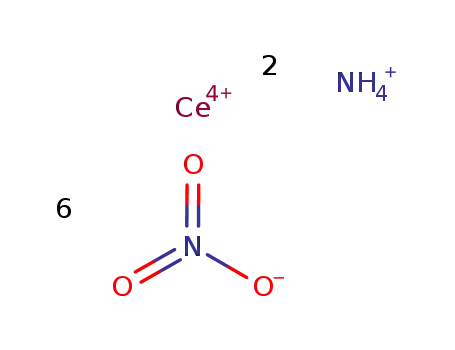
Product Details
CAS: 16774-21-3
MF: CeH4N7O18-
Appearance: yellow-orange crystals
Packing: 25kg/ bag, 50kg/ bag
Purity: CeO2/TREO≥99.99%
High Purity Reputable Factory Supply Cerium Ammonium nitrate 16774-21-3 with Safe Transportation
- Molecular Formula:CeN8H8O6N18
- Molecular Weight:530.18
- Appearance/Colour:yellow-orange crystals
- Vapor Pressure:49.8mmHg at 25°C
- Melting Point:107-108 °C
- Boiling Point:83oC at 760 mmHg
- Flash Point:87oC
- PSA:414.10000
- Density:1.10 g/cm3
- LogP:2.13520
Ceric ammonium nitrate(Cas 16774-21-3) Usage
|
Physical properties
|
Reddish-orange monoclinic crystals; very soluble in water.
|
|
Preparation
|
Ceric ammonium nitrate is prepared by electrolytic oxidation of cerous nitrate in nitric acid to ceric nitrate, followed by the addition of ammonium nitrate solution. It is separated from the solution by crystallization. It may be prepared alternatively by dissolving cerium(II) oxide, CeO?H2O in concentrated nitric acid followed by treatment with ammonium nitrate.
|
|
Reactions
|
The most important reactions of Ceric ammonium nitrate are the oxidations, attributed to Ce4+ ion in the solution. The standard reduction potential E° for the formal half-reaction: Ce4+ + e– Ce3+ in 1 M H2SO4 is 1.44 V. The oxidizing strength is comparable to permanganate (MnO4- ), bromate (BrO3-), and dichromate (Cr2O72-) anions. Analytical applications involve reactions with reductants such as sodium oxalate (Na2C2O4) or arsenic (III) oxide (As2O3) in the presence of iron, with ferroin (1,10–phenanthroline iron(II) complex) as the indicator.
|
|
General Description
|
Ammonium cerium(IV) nitrate (Cerium(IV) ammonium nitrate, CAN) is a versatile reagent for oxidative electron transfer reactions. It participates in various novel carbon-carbon bond-forming reactions involved in one-pot synthesis of dihydrofurans, tetrahydrofurans and aminotetralins. Iodine/ammonium cerium(IV) nitrate has been employed in direct α-iodination of various ketones to afford the corresponding α-iodo ketones. CAN has been reported as an powerful one-electron oxidant. It also participates in various carbon-heteroatom bond-forming reactions.
|
|
Hazard
|
Ceric ammonium nitrate is a powerful oxidizing agent. Precautions should be taken to avoid accidental contacts with orgnaic or other readily oxidizable substances.
|
|
Flammability and Explosibility
|
Notclassified
|
|
Purification Methods
|
Ceric ammonium nitrate (125g) is warmed with 100mL of dilute HNO3 (1:3 v/v) and 40g of NH4NO3 until it dissolves, and filtered through a sintered-glass funnel. The solid which separates on cooling in ice is filtered off on a sintered funnel (at the pump) and air is sucked through the solid for 1-2 hours to remove most of the nitric acid. Finally, the solid is dried at 80-85o.
|
InChI:InChI=1/Ce.NO3.H3N/c;2-1(3)4;/h;;1H3/q+4;-1;/p+1
16774-21-3 Relevant articles
Electrokinetic properties and stability of cerium dioxide suspensions
Barany,Bohacs,Chepurna,Meszaros
, p. 69343 - 69351 (2016/08/05)
The dispersed ceria particles synthesize...
16774-21-3 Process route
-
- 16774-21-3
ammonium cerium (IV) nitrate
Conditions
| Conditions |
Yield |
|
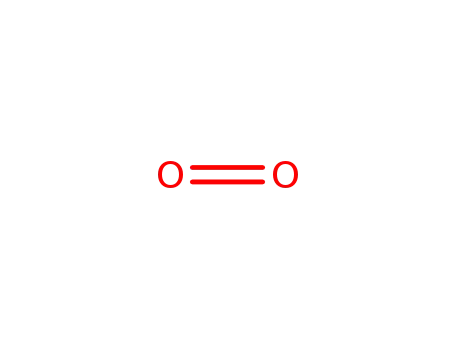
With dihydrogen peroxide;
|
|
-
- 16774-21-3
ammonium cerium (IV) nitrate
-
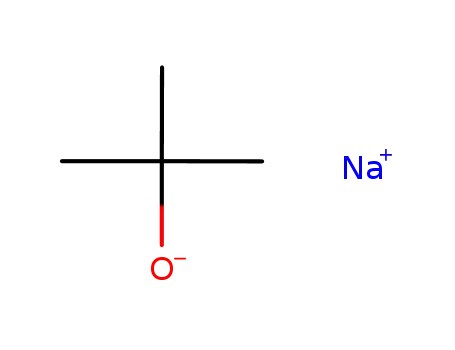
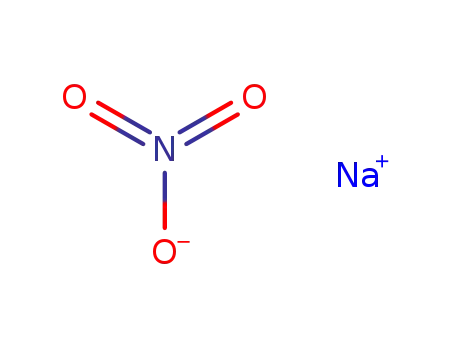
- 865-48-5
sodium t-butanolate
-
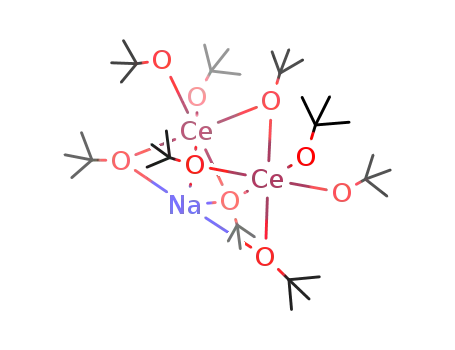
- 122423-62-5
Ce2(OCMe3)4(μ-OCMe3)3(μ3-OCMe3)2Na
-
- 75-65-0
tert-butyl alcohol
Conditions
| Conditions |
Yield |
|
In tetrahydrofuran; byproducts: NH3; exclusion of air and water, stirred for 2 h; filtered (NaNO3), removal of solvent from filtrate, extn. (hexane), removal of solvent; elem. anal.;
|
96%
94% |
16774-21-3 Downstream products