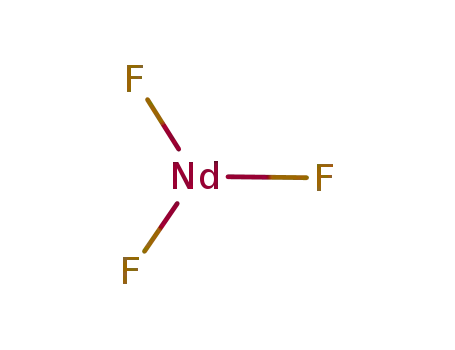
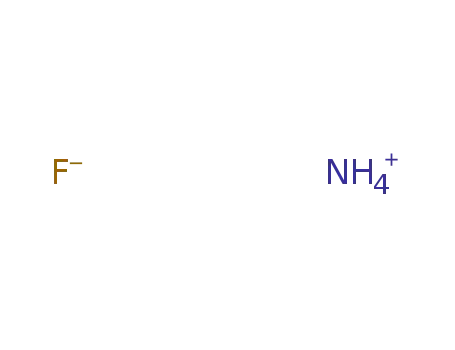
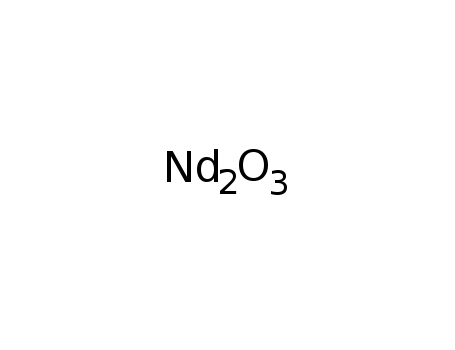
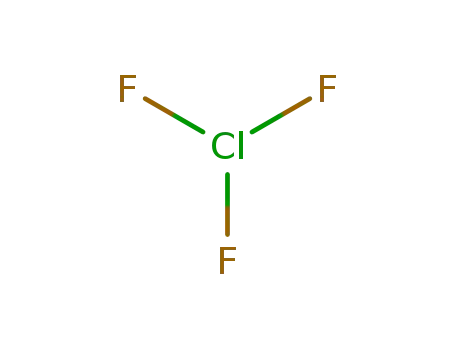
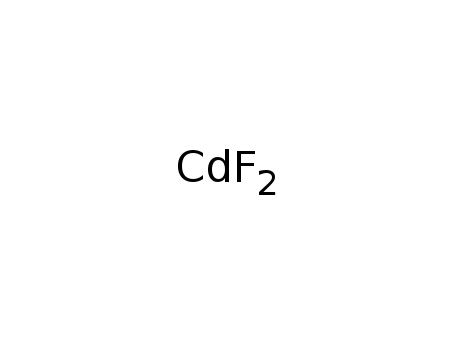
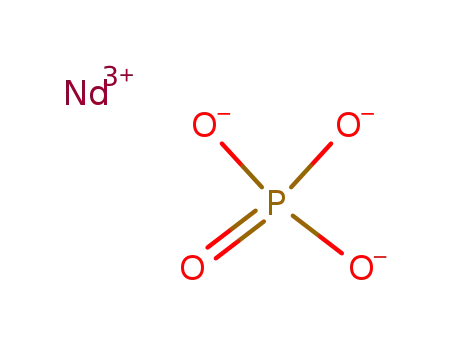
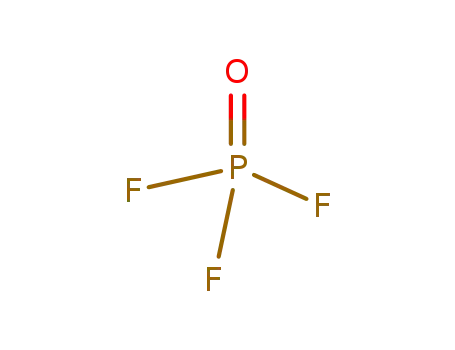
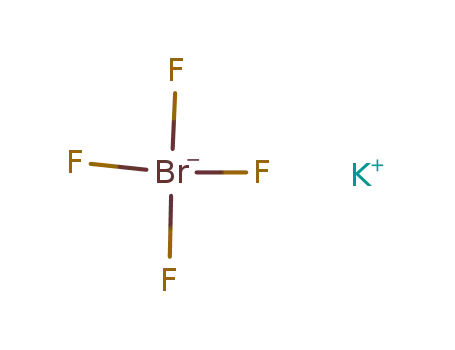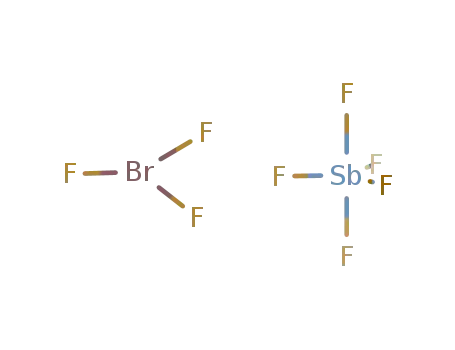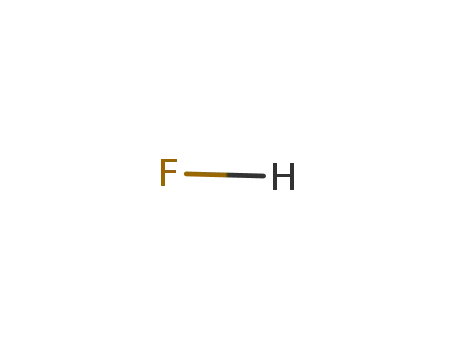Phase analyses of lanthanide oxide fluorides
Shinn, Dennis B.,Eick, Harry A.
, p. 232 - 235 (1969)
Approximate composition limits and latti...
Synthesis and characterization of Ba1-xNdxF2+x (0.00 ≤ x ≤ 1.00)
Grover,Achary,Patwe,Tyagi
, p. 1101 - 1111 (2003)
A series of mixed fluorides with general...
Laser development of rare-earth doped crystals
Vieira Jr.,Ranieri,Tarelho,Wetter,Baldochi,Gomes,De Matos,De Rossi,Nogueira,Courrol,Barbosa,Maldonado,Morato
, p. 231 - 239 (2002)
Rare earth doped laser crystals present ...
Heterometallic Ln/Hg compounds with fluorinated thiolate ligands
Banerjee, Santanu,Emge, Thomas J.,Brennan, John G.
, p. 6307 - 6312 (2004)
The early lanthanide benzenefluorothiola...
Solubility of YF3, CeF3, PrF3, NdF 3, and DyF3 in solutions containing sulfuric and phosphoric acids
Lokshin,Tareeva
, p. 1830 - 1834 (2007)
The solubility of YF3, CeF3, PrF3, NdF3,...
Spectroscopic characterization of Ho3+ ion-doped fluoride glass
Florez,Oliveira,Flórez,Gómez,Nunes
, p. 238 - 242 (2006)
Among the new optical materials availabl...
Optical properties of Nd3+-doped fluorophosphate glasses
Binnemans,Van Deun,Gorller-Walrand,Adam
, p. 455 - 460 (1998)
Optical absorption spectra of Nd3+-doped...
Synthesis and characterization of LnF(HF)(BF4)2 (Ln = La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, and Dy), and crystal structures of LnF(HF)(BF4)2 (Ln = Pr, Nd) and La(BF4) 3
Mazej, Zoran,Goreshnik, Evgeny,Hironaka, Kohei,Katayama, Yasushi,Hagiwara, Rika
, p. 2309 - 2315 (2009)
Rare earth trifluorides (Ln = La, Ce, Pr...
Hydrofluoride synthesis of fluorides of some rare-earth elements
Kalinnikov,Makarov,Tikhomirova,Elizarova,Kuznetsov
, p. 1760 - 1764 (2002)
Thermal gravimetric, X-ray phase, IR spe...
High-quality sodium rare-earth fluoride nanocrystals: Controlled synthesis and optical properties
Mai, Hao-Xin,Zhang, Ya-Wen,Si, Rui,Yan, Zheng-Guang,Sun, Ling-Dong,You, Li-Ping,Yan, Chun-Hua
, p. 6426 - 6436 (2006)
We report a general synthesis of high-qu...
Phase relation studies in Pb1-xM′xF 2+x systems (0.0≤x≤1.0; M′=Nd3+, Eu 3+ and Er3+)
Tyagi,Patwe,Achary,Mallia
, p. 1746 - 1757 (2004)
In this communication we report the synt...
Lanthanide pentafluorophenolates. Synthesis, structure and luminescent properties
Maleev, Alexander A.,Fagin, Anatoly A.,Ilichev, Vasily A.,Lopatin, Mikhail A.,Konev, Alexey N.,Samsonov, Maksim A.,Fukin, Georgy K.,Bochkarev, Mikhail N.
, p. 126 - 132 (2013/11/19)
The pentafluorophenolates of lanthanides...
X-ray magnetic circular dichroism (XMCD) study of a methoxide-bridged DyIII-CrIII cluster obtained by fluoride abstraction from cis-[CrIIIF2(phen)2]+
Dreiser, Jan,Pedersen, Kasper S.,Birk, Torben,Schau-Magnussen, Magnus,Piamonteze, Cinthia,Rusponi, Stefano,Weyhermueller, Thomas,Brune, Harald,Nolting, Frithjof,Bendix, Jesper
, p. 7842 - 7847 (2012/09/22)
An isostructural series of dinuclear chr...
Infrared spectra and quantum chemical calculations of the bridge-bonded HC(F)LnF2 (Ln = La-Lu) complexes
Gong, Yu,Wang, Xuefeng,Andrews, Lester,Chen, Mingyang,Dixon, David A.
, p. 4443 - 4452 (2011/10/10)
Lanthanide metal atoms, produced by lase...
The versatility of solid-state metathesis reactions: From rare earth fluorides to carboiimides
Unverfehrt, Leonid,Glaser, Jochen,Stroebele, Markus,Tragl, Sonja,Gibson, Katharina,Meyer, H.-Juergen
, p. 479 - 483 (2009/06/17)
The new carbodiimide compounds LaF(CN2) ...